CYP17A1 Rabbit mAb [L906]Cat NO.: A53140
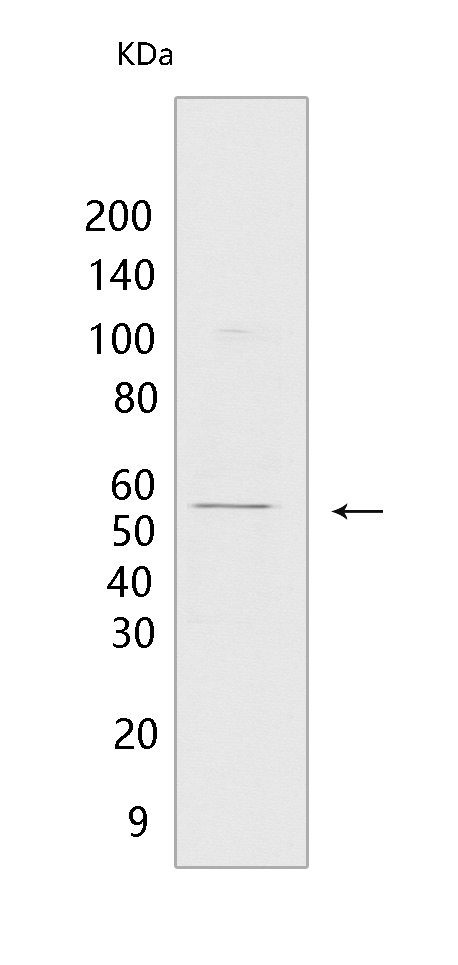
Western blot(SDS PAGE) analysis of extracts from Mouse liver.Using CYP17A1 Rabbit mAb [L906] at dilution of 1:1000 incubated at 4℃ over night.
Product information
Protein names :CYP17A1,CYP17,S17AH,CP17A_HUMAN,Steroid 17-alpha-hydroxylase/17,20 lyase
UniProtID :P05093
MASS(da) :57,371
MW(kDa) :55 kDa
Form :Liquid
Purification :Protein A purification
Host :Rabbit
Isotype :IgG
sensitivity :Endogenous
Reactivity :Human,Mouse,Rat
- ApplicationDilution
- 免疫印迹(WB)1:1000-2000
- The optimal dilutions should be determined by the end user
Specificity :Antibody is produced by immunizing animals with a synthetic peptide at the sequence of Human CYP17A1
Storage :Antibody store in 10 mM PBS, 0.5mg/ml BSA, 50% glycerol. Shipped at 4°C. Store at-20°C or -80°C. Products are valid for one natural year of receipt.Avoid repeated freeze / thaw cycles.
WB Positive detected :Mouse liver
Function : A cytochrome P450 monooxygenase involved in corticoid and androgen biosynthesis (PubMed:9452426, PubMed:27339894, PubMed:22266943, PubMed:25301938). Catalyzes 17-alpha hydroxylation of C21 steroids, which is common for both pathways. A second oxidative step, required only for androgen synthesis, involves an acyl-carbon cleavage. The 17-alpha hydroxy intermediates, as part of adrenal glucocorticoids biosynthesis pathway, are precursors of cortisol (PubMed:9452426, PubMed:25301938) (Probable). Hydroxylates steroid hormones, pregnenolone and progesterone to form 17-alpha hydroxy metabolites, followed by the cleavage of the C17-C20 bond to form C19 steroids, dehydroepiandrosterone (DHEA) and androstenedione (PubMed:9452426, PubMed:27339894, PubMed:22266943, PubMed:25301938). Has 16-alpha hydroxylase activity. Catalyzes 16-alpha hydroxylation of 17-alpha hydroxy pregnenolone, followed by the cleavage of the C17-C20 bond to form 16-alpha-hydroxy DHEA. Also 16-alpha hydroxylates androgens, relevant for estriol synthesis (PubMed:27339894, PubMed:25301938). Mechanistically, uses molecular oxygen inserting one oxygen atom into a substrate, and reducing the second into a water molecule, with two electrons provided by NADPH via cytochrome P450 reductase (CPR,NADPH-ferrihemoprotein reductase) (PubMed:9452426, PubMed:27339894, PubMed:22266943, PubMed:25301938)..
Subcellular locationi :Endoplasmic reticulum membrane. Microsome membrane.
IMPORTANT: For western blots, incubate membrane with diluted primary antibody in 1% w/v BSA, 1X TBST at 4°C overnight.