Hsp60 Rabbit mAb [C8MH]Cat NO.: A84243
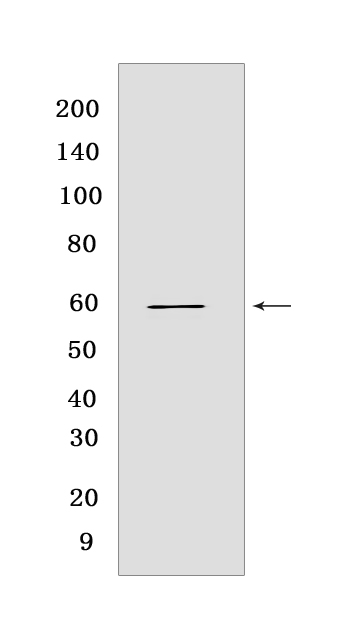
Western blot(SDS PAGE) analysis of extracts from T47D cells .Using Hsp60Rabbit mAb [C8MH] at dilution of 1:1000 incubated at 4℃ over night.
Product information
Protein names :HSPD1,HSP60,CH60_HUMAN,60 kDa heat shock protein, mitochondrial
UniProtID :P10809
MASS(da) :61,055
MW(kDa) :60 kDa
Form :Liquid
Purification :Protein A purification
Host :Rabbit
Isotype :IgG
sensitivity :Endogenous
Reactivity :Human,Mouse,Rat
- ApplicationDilution
- 免疫印迹(WB)1:1000-2000
- 免疫组化(IHC)1:100
- The optimal dilutions should be determined by the end user
Specificity :Antibody is produced by immunizing animals with a synthetic peptide at the sequence of human Hsp60
Storage :Antibody store in 10 mM PBS, 0.5mg/ml BSA, 50% glycerol. Shipped at 4°C. Store at-20°C or -80°C. Products are valid for one natural year of receipt.Avoid repeated freeze / thaw cycles.
WB Positive detected :T47D cells
Function : Chaperonin implicated in mitochondrial protein import and macromolecular assembly. Together with Hsp10, facilitates the correct folding of imported proteins. May also prevent misfolding and promote the refolding and proper assembly of unfolded polypeptides generated under stress conditions in the mitochondrial matrix (PubMed:1346131, PubMed:11422376). The functional units of these chaperonins consist of heptameric rings of the large subunit Hsp60, which function as a back-to-back double ring. In a cyclic reaction, Hsp60 ring complexes bind one unfolded substrate protein per ring, followed by the binding of ATP and association with 2 heptameric rings of the co-chaperonin Hsp10. This leads to sequestration of the substrate protein in the inner cavity of Hsp60 where, for a certain period of time, it can fold undisturbed by other cell components. Synchronous hydrolysis of ATP in all Hsp60 subunits results in the dissociation of the chaperonin rings and the release of ADP and the folded substrate protein (Probable)..
Subcellular locationi :Mitochondrion matrix.
IMPORTANT: For western blots, incubate membrane with diluted primary antibody in 1% w/v BSA, 1X TBST at 4°C overnight.