HSP90AB1 Rabbit mAb [5801]Cat NO.: A41627
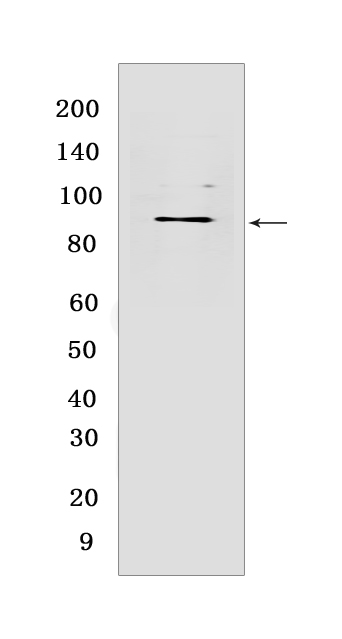
Western blot(SDS PAGE) analysis of extracts from LNCaP cells .Using HSP90AB1 Rabbit mAb [5801] at dilution of 1:1000 incubated at 4℃ over night.
Product information
Protein names :HSP90AB1,HSP90B,HSPC2,HSPCB,HS90B_HUMAN,Heat shock protein HSP 90-beta
UniProtID :P08238
MASS(da) :83,264
MW(kDa) :90
Form :Liquid
Purification :Protein A purification
Host :Rabbit
Isotype :IgG
sensitivity :Endogenous
Reactivity :Human,Mouse,Rat
- ApplicationDilution
- 免疫印迹(WB)1:1000-2000,
- 免疫组化(IHC)1:100
- The optimal dilutions should be determined by the end user
Specificity :Antibody is produced by immunizing animals with a synthetic peptide at the sequence of human HSP90AB1.
Storage :Antibody store in 10 mM PBS, 0.5mg/ml BSA, 105% glycerol. Shipped at 4°C. Store at-20°C or -80°C. Products are valid for one natural year of receipt.Avoid repeated freeze / thaw cycles.
WB Positive detected :LNCaP cells
Function : Molecular chaperone that promotes the maturation, structural maintenance and proper regulation of specific target proteins involved for instance in cell cycle control and signal transduction. Undergoes a functional cycle linked to its ATPase activity. This cycle probably induces conformational changes in the client proteins, thereby causing their activation. Interacts dynamically with various co-chaperones that modulate its substrate recognition, ATPase cycle and chaperone function (PubMed:16478993, PubMed:19696785). Engages with a range of client protein classes via its interaction with various co-chaperone proteins or complexes, that act as adapters, simultaneously able to interact with the specific client and the central chaperone itself. Recruitment of ATP and co-chaperone followed by client protein forms a functional chaperone. After the completion of the chaperoning process, properly folded client protein and co-chaperone leave HSP90 in an ADP-bound partially open conformation and finally, ADP is released from HSP90 which acquires an open conformation for the next cycle (PubMed:27295069, PubMed:26991466). Apart from its chaperone activity, it also plays a role in the regulation of the transcription machinery. HSP90 and its co-chaperones modulate transcription at least at three different levels. They first alter the steady-state levels of certain transcription factors in response to various physiological cues. Second, they modulate the activity of certain epigenetic modifiers, such as histone deacetylases or DNA methyl transferases, and thereby respond to the change in the environment. Third, they participate in the eviction of histones from the promoter region of certain genes and thereby turn on gene expression (PubMed:25973397). Antagonizes STUB1-mediated inhibition of TGF-beta signaling via inhibition of STUB1-mediated SMAD3 ubiquitination and degradation (PubMed:24613385). Promotes cell differentiation by chaperoning BIRC2 and thereby protecting from auto-ubiquitination and degradation by the proteasomal machinery (PubMed:18239673). Main chaperone involved in the phosphorylation/activation of the STAT1 by chaperoning both JAK2 and PRKCE under heat shock and in turn, activates its own transcription (PubMed:20353823). Involved in the translocation into ERGIC (endoplasmic reticulum-Golgi intermediate compartment) of leaderless cargos (lacking the secretion signal sequence) such as the interleukin 1/IL-1,the translocation process is mediated by the cargo receptor TMED10 (PubMed:32272059).., (Microbial infection) Binding to N.meningitidis NadA stimulates monocytes (PubMed:21949862). Seems to interfere with N.meningitidis NadA-mediated invasion of human cells (Probable)..
Subcellular locationi :Cytoplasm. Melanosome. Nucleus. Secreted. Cell membrane. Dynein axonemal particle. Cell surface.
IMPORTANT: For western blots, incubate membrane with diluted primary antibody in 1% w/v BSA, 1X TBST at 4°C overnight.