STUB1/CHIP Rabbit mAb [MWY3]Cat NO.: A40091
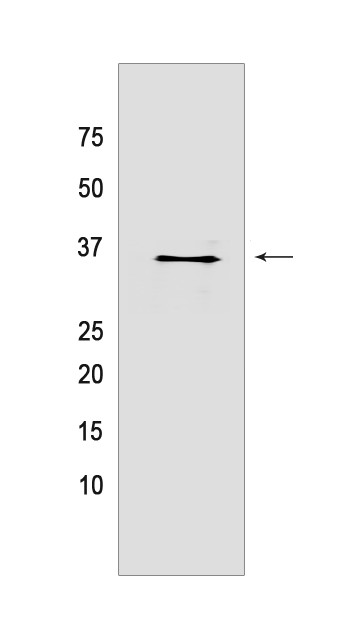
Western blot analysis of extracts from HeLa cells lyastes.using STUB1/CHIP Rabbit mAb [MWY3] at dilution of 1:1000 incubated at 4℃ over night
Product information
Protein names :STUB1,CHIP,PP1131,CHIP_HUMAN,E3 ubiquitin-protein ligase CHIP
UniProtID :Q9UNE7
MASS(da) :34,856
MW(kDa) :35 kDa
Form :Liquid
Purification :Protein A purification
Host :Rabbit
Isotype :IgG
sensitivity :Endogenous
Reactivity :Human
- ApplicationDilution
- 免疫印迹(WB)1:1000-2000
- 免疫荧光(ICC/IF)1:100,
- The optimal dilutions should be determined by the end user
Specificity :Antibody is produced by immunizing animals with a synthetic peptide of Human STUB1/CHIP.
Storage :Antibody store in 10 mM PBS, 0.5mg/ml BSA, 50% glycerol. Shipped at 4°C. Store at-20°C or -80°C. Products are valid for one natural year of receipt.Avoid repeated freeze / thaw cycles.
WB Positive detected :HeLa cells lyastes
Function : E3 ubiquitin-protein ligase which targets misfolded chaperone substrates towards proteasomal degradation (PubMed:10330192, PubMed:11146632, PubMed:11557750, PubMed:23990462). Collaborates with ATXN3 in the degradation of misfolded chaperone substrates: ATXN3 restricting the length of ubiquitin chain attached to STUB1/CHIP substrates and preventing further chain extension (PubMed:10330192, PubMed:11146632, PubMed:11557750, PubMed:23990462). Ubiquitinates NOS1 in concert with Hsp70 and Hsp40 (PubMed:15466472). Modulates the activity of several chaperone complexes, including Hsp70, Hsc70 and Hsp90 (PubMed:10330192, PubMed:11146632, PubMed:15466472). Mediates transfer of non-canonical short ubiquitin chains to HSPA8 that have no effect on HSPA8 degradation (PubMed:11557750, PubMed:23990462). Mediates polyubiquitination of DNA polymerase beta (POLB) at 'Lys-41', 'Lys-61' and 'Lys-81', thereby playing a role in base-excision repair: catalyzes polyubiquitination by amplifying the HUWE1/ARF-BP1-dependent monoubiquitination and leading to POLB-degradation by the proteasome (PubMed:19713937). Mediates polyubiquitination of CYP3A4 (PubMed:19103148). Ubiquitinates EPHA2 and may regulate the receptor stability and activity through proteasomal degradation (PubMed:19567782). Acts as a co-chaperone for HSPA1A and HSPA1B chaperone proteins and promotes ubiquitin-mediated protein degradation (PubMed:27708256). Negatively regulates the suppressive function of regulatory T-cells (Treg) during inflammation by mediating the ubiquitination and degradation of FOXP3 in a HSPA1A/B-dependent manner (PubMed:23973223). Catalyzes monoubiquitination of SIRT6, preventing its degradation by the proteasome (PubMed:24043303). Likely mediates polyubiquitination and down-regulates plasma membrane expression of PD-L1/CD274, an immune inhibitory ligand critical for immune tolerance to self and antitumor immunity (PubMed:28813410). Negatively regulates TGF-beta signaling by modulating the basal level of SMAD3 via ubiquitin-mediated degradation (PubMed:24613385). May regulate myosin assembly in striated muscles together with UBE4B and VCP/p97 by targeting myosin chaperone UNC45B for proteasomal degradation (PubMed:17369820). Mediates ubiquitination of RIPK3 leading to its subsequent proteasome-dependent degradation (PubMed:29883609)..
Tissue specificity :Expressed in differentiated myotubes (at protein level) (PubMed:17369820). Highly expressed in skeletal muscle, heart, pancreas, brain and placenta (PubMed:10330192, PubMed:11435423). Detected in kidney, liver and lung (PubMed:10330192, PubMed:11435423)..
Subcellular locationi :Cytoplasm. Nucleus.
IMPORTANT: For western blots, incubate membrane with diluted primary antibody in 1% w/v BSA, 1X TBST at 4°C overnight.