Cytochrome P450 1A2 Rabbit mAb [4KB0]Cat NO.: A30523
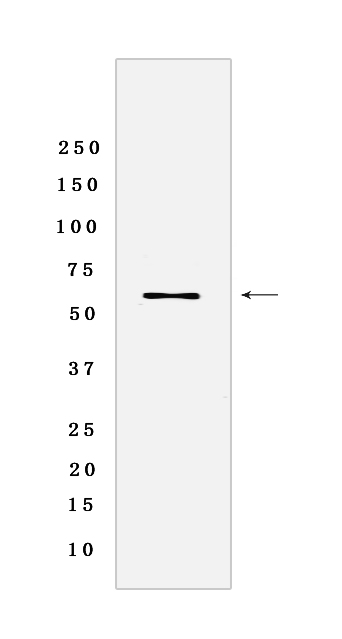
Western blot analysis of extracts from Caco2 cells lyastes.using Cytochrome P450 1A2 Rabbit mAb [4KB0] at dilution of 1:1000 incubated at 4℃ over night
Product information
Protein names :CYP1A2,CP1A2_HUMAN,Cytochrome P450 1A2 450)
UniProtID :P05177
MASS(da) :58,407
MW(kDa) :58 kDa
Form :Liquid
Purification :Protein A purification
Host :Rabbit
Isotype :IgG
sensitivity :Endogenous
Reactivity :Human
- ApplicationDilution
- 免疫印迹(WB)1:1000-2000
- 免疫荧光(ICC/IF)1:100,
- The optimal dilutions should be determined by the end user
Specificity :Antibody is produced by immunizing animals with a synthetic peptide of Human Cytochrome P450 1A2.
Storage :Antibody store in 10 mM PBS, 0.5mg/ml BSA, 50% glycerol. Shipped at 4°C. Store at-20°C or -80°C. Products are valid for one natural year of receipt.Avoid repeated freeze / thaw cycles.
WB Positive detected :Caco2 cells lyastes
Function : A cytochrome P450 monooxygenase involved in the metabolism of various endogenous substrates, including fatty acids, steroid hormones and vitamins (PubMed:9435160, PubMed:10681376, PubMed:11555828, PubMed:12865317, PubMed:19965576). Mechanistically, uses molecular oxygen inserting one oxygen atom into a substrate, and reducing the second into a water molecule, with two electrons provided by NADPH via cytochrome P450 reductase (NADPH--hemoprotein reductase) (PubMed:9435160, PubMed:10681376, PubMed:11555828, PubMed:12865317, PubMed:19965576). Catalyzes the hydroxylation of carbon-hydrogen bonds (PubMed:11555828, PubMed:12865317). Exhibits high catalytic activity for the formation of hydroxyestrogens from estrone (E1) and 17beta-estradiol (E2), namely 2-hydroxy E1 and E2 (PubMed:11555828, PubMed:12865317). Metabolizes cholesterol toward 25-hydroxycholesterol, a physiological regulator of cellular cholesterol homeostasis (PubMed:21576599). May act as a major enzyme for all-trans retinoic acid biosynthesis in the liver. Catalyzes two successive oxidative transformation of all-trans retinol to all-trans retinal and then to the active form all-trans retinoic acid (PubMed:10681376). Primarily catalyzes stereoselective epoxidation of the last double bond of polyunsaturated fatty acids (PUFA), displaying a strong preference for the (R,S) stereoisomer (PubMed:19965576). Catalyzes bisallylic hydroxylation and omega-1 hydroxylation of PUFA (PubMed:9435160). May also participate in eicosanoids metabolism by converting hydroperoxide species into oxo metabolites (lipoxygenase-like reaction, NADPH-independent) (PubMed:21068195). Plays a role in the oxidative metabolism of xenobiotics. Catalyzes the N-hydroxylation of heterocyclic amines and the O-deethylation of phenacetin (PubMed:14725854). Metabolizes caffeine via N3-demethylation (Probable)..
Tissue specificity :Liver.
Subcellular locationi :Endoplasmic reticulum membrane,Peripheral membrane protein. Microsome membrane,Peripheral membrane protein.
IMPORTANT: For western blots, incubate membrane with diluted primary antibody in 1% w/v BSA, 1X TBST at 4°C overnight.