Hsp90 Rabbit mAb [nxeS]Cat NO.: A95000
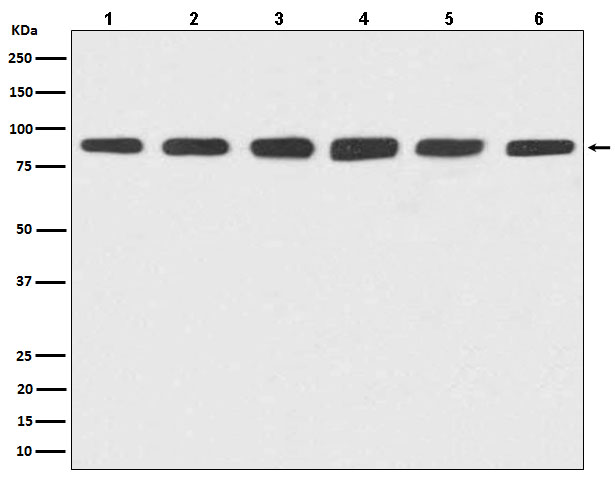
Western blot(SDS PAGE) analysis of extracts from (1) HeLa cell lysate; (2) Jurkat cell lysate; (3) RAW264.7 cell lysate; (4) NIH/3T3 cell lysate; (5) PC-12 cell lysate; (6) C6 cell lysate.Using Hsp90 Rabbit mAb [nxeS]at dilution of 1:1000 incubated at 4℃ over night.
Product information
Protein names :HSP 90; HSP90A; HSP90B; LAP-2; HSPC1; HSPC2;
UniProtID :P07900/P08238
MASS(da) :84,660
MW(kDa) :90kDa
Form :Liquid
Purification :Affinity-chromatography
Host :Rabbit
Isotype : IgG
sensitivity :Endogenous
Reactivity :Human,Mouse,Rat
- ApplicationDilution
- 免疫印迹(WB)1:1000-2000
- 免疫组化(IHC)1:100
- 免疫荧光(ICC/IF)1:100
- The optimal dilutions should be determined by the end user
Specificity :Antibody is produced by immunizing animals with A synthesized peptide derived from human Hsp90
Storage :Antibody store in 10 mM PBS, 0.5mg/ml BSA, 50% glycerol. Shipped at 4°C. Store at-20°C or -80°C. Products are valid for one natural year of receipt.Avoid repeated freeze / thaw cycles.
WB Positive detected :(1) HeLa cell lysate; (2) Jurkat cell lysate; (3) RAW264.7 cell lysate; (4) NIH/3T3 cell lysate; (5) PC-12 cell lysate; (6) C6 cell lysate.
Function : Molecular chaperone that promotes the maturation, structural maintenance and proper regulation of specific target proteins involved for instance in cell cycle control and signal transduction. Undergoes a functional cycle that is linked to its ATPase activity which is essential for its chaperone activity. This cycle probably induces conformational changes in the client proteins, thereby causing their activation. Interacts dynamically with various co-chaperones that modulate its substrate recognition, ATPase cycle and chaperone function (PubMed:11274138, PubMed:15577939, PubMed:15937123, PubMed:27353360, PubMed:29127155, PubMed:12526792). Engages with a range of client protein classes via its interaction with various co-chaperone proteins or complexes, that act as adapters, simultaneously able to interact with the specific client and the central chaperone itself (PubMed:29127155). Recruitment of ATP and co-chaperone followed by client protein forms a functional chaperone. After the completion of the chaperoning process, properly folded client protein and co-chaperone leave HSP90 in an ADP-bound partially open conformation and finally, ADP is released from HSP90 which acquires an open conformation for the next cycle (PubMed:27295069, PubMed:26991466). Plays a critical role in mitochondrial import, delivers preproteins to the mitochondrial import receptor TOMM70 (PubMed:12526792). Apart from its chaperone activity, it also plays a role in the regulation of the transcription machinery. HSP90 and its co-chaperones modulate transcription at least at three different levels (PubMed:25973397). In the first place, they alter the steady-state levels of certain transcription factors in response to various physiological cues(PubMed:25973397). Second, they modulate the activity of certain epigenetic modifiers, such as histone deacetylases or DNA methyl transferases, and thereby respond to the change in the environment (PubMed:25973397). Third, they participate in the eviction of histones from the promoter region of certain genes and thereby turn on gene expression (PubMed:25973397). Binds bacterial lipopolysaccharide (LPS) and mediates LPS-induced inflammatory response, including TNF secretion by monocytes (PubMed:11276205). Antagonizes STUB1-mediated inhibition of TGF-beta signaling via inhibition of STUB1-mediated SMAD3 ubiquitination and degradation (PubMed:24613385). Mediates the association of TOMM70 with IRF3 or TBK1 in mitochondrial outer membrane which promotes host antiviral response (PubMed:20628368, PubMed:25609812).., (Microbial infection) Seems to interfere with N.meningitidis NadA-mediated invasion of human cells. Decreasing HSP90 levels increases adhesion and entry of E.coli expressing NadA into human Chang cells,increasing its levels leads to decreased adhesion and invasion..
Subcellular locationi :Nucleus. Cytoplasm. Melanosome. Cell membrane. Mitochondrion.
IMPORTANT: For western blots, incubate membrane with diluted primary antibody in 1% w/v BSA, 1X TBST at 4°C overnight.